Understanding Technology Transfer Regulations In India

Toretain or strengthen a business, and competitive position in the market, new technology invention or adoption has become quintessential. Companies in industries like the extraction or commercialization of raw materials may rely on new technologies to increase the efficiency of their raw material extraction through the improvement of their production processes or the acquisition of new machinery and equipment. Companies also leverage modern technology to enhance management structure, control, and communication, or to effectively market their products. Technological Innovation is an essential component of every company’s competitive strategy.
Technology Transfer refers to the transfer of a technology, knowledge, know-how, or facility from one person, business, or organization to another. It enables partners to share their expertise to expand into new markets, commercialise a new good or service, or enhance an already existing good or process, and to sell their goods/services swiftly.
The transfer of the exclusive rights to a patented technology or the permission to use a specific technology or know-how occurs through the establishment of legal relationships between the “transferor,” who is the owner of the exclusive rights or the supplier of the know-how, and the “transferee,” who purchases them.
Table of Contents
Importance
Technology Transfers and their agreements are important as –
1. They strengthen commercial ties
2. TT Agreements make a legally-binding, contractual agreement that is enforced by both parties.
3. Handle intellectual property, including ownership, licencing, and royalties (such as patents, copyrights, trademarks, and industrial designs).
4. Help in accessing technology
5. Provide adaptable protection for one or more categories of intellectual property rights.
6. Resolve disputes over contracts by referring to the relevant terms included in the Technology Transfer agreement and provisions of Technology Transfer Regulations valid in India,
7. Enable the non-licensing transfer of technology pertaining to methods, know-how, and trade secrets.
8. Identify the goal of technology transfer – creation of new goods, expansion into new markets, legal and tax implications, and litigation rights.
Technology Transfer Regulations In India
The Technology Transfer Regulations in India are as follows: –
- Indian Contract Act, 1972
- It is the only comprehensive legislation in India that governs contracts. There are rules that are tailored to suit the nature of agreement.
- Foreign Exchange Management Act
- Rule 4 of the Foreign Exchange Management (Current Account Transactions) Rules 2000 (“Rules”) states that the Ministry of Commerce and Industry of the Government of India must first approve any withdrawals of foreign currency for remittances made in connection with technical collaboration agreements where the royalty payment exceeds 5% on domestic sales and 8% on exports and the lump-sum payment exceeds USD 2 million[1]. Item no. 8 of the Rules and the entry pertaining to it were determined to be excluded after the Government of India evaluated the current policy regarding the liberalisation of foreign technology agreements. Thus, AD Category-I banks may authorise foreign currency withdrawals by individuals for the purpose of paying royalties and lump sums under technical partnership agreements without the consent of the Ministry of Commerce and Industry.
- Intellectual Property Rights
- The Patents Act, 1970, the Trademarks Act, 1999, and the Copyright Act, 1957 all govern and protect the intellectual property rights in India. They specify the procedures for transferring IPRs and form a part of Technology Transfer Regulations in India.
- According to the Patents Act, any interest in a patent, including an assignment or licence, must be through a written document that contains all the terms and conditions governing the parties’ rights and obligations. This document must be registered with the Controller of the Patents.
- A registered trademark may be transferred/assigned under the Act with or without the goodwill of the business. To prove ownership of the registered mark, the assignment must be registered.
- The Copyright Act states that an author may grant his rights to third parties for commercial exploitation in exchange for a one-time payment. Copyright assignments must be in writing and signed by the assignor. The deed of assignment shall include the identity of the work, the rights granted, the term, and the territorial scope of such an assignment, as well as the amount of any royalties due to the author.
- The National Intellectual Property Rights Policy
- The National Intellectual Property Rights Policy aims to strengthen the country’s IPR framework by raising public awareness of the economic, social, and cultural benefits of IPRs among all societal segments, promoting IPR generation and commercialization, modernising and strengthening service oriented IPR administration, and strengthening the enforcement and adjudicatory mechanisms for dealing with IPR violations.
- The National Intellectual Property Rights Policy outlines seven objectives that are further defined with steps that must be implemented by the designated nodal Ministry/Department. The objectives include the following steps that are taken in relation to Technology Transfer Regulations in India: –
- Undertake studies to assess the contribution of IP content in different industries on the economy, employment, exports and technology transfer.
- Examine the issues of technology transfer, know-how and licensing relating to SEPs on fair and reasonable terms and provide asuitable legal framework to address these issues, as may be required.
- Promote licensing and technology transfer for IPRs; devising suitable contractual and licensing guidelines to enable commercialization of IPRs; promote patent pooling and cross-licensing to create IPR based products and services.[2]
- Competition Act, 2002
- The Act establishes the Competition Commission of India which, is tasked with outlawing anticompetitive agreements that have the potential to cause appreciable adverse effects on competition in markets and prohibits abuse of dominance by enterprises. According to Section 3(5)(a) to (f) of the Act, a technology owner is fully entitled to prevent any violation of his rights and to apply reasonable restrictions that are only required to safeguard those rights.
Technology Transfer Agreements (“TTA”) would be deemed anti-competitive if they result in the abuse of a market position by imposing unreasonable terms or if they go beyond what is necessary to maintain such intellectual property rights. The following technology transfer agreements may be deemed anti-competitive and thus null and void:
- Patent Pooling, in which two or more businesses join and cross licence the relevant technology to prevent others from purchasing it.
- Tie in Arrangements that require the acquirer to purchase both the patented product and the other product from the patentee.
- Forbidding the licensee from using technology from a competing enterprise.
- Restricting the licensee’s ability to dispute the legality of intellectual property rights.
- Fixing the price at which the licensee will sell the licenced goods, etc.
Guidelines for Transfer of Technology in Pharmaceutical Sector
Technology transfer involves a planned, organised strategy with data documentation that covers all stages of development, manufacturing, and quality control, as well as educated, competent individuals operating within a quality system. Sending units (SU), receiving units, and the unit overseeing the processare often included in a system.
Technology transfer is essential to the process of discovering novel drugs and developing new pharmaceuticals. Data gathering, data evaluation, regulatory impact, with a focus on any modification approvals, analytical validation, pilot or full-scale process batch, and stability set down are important steps of the process.
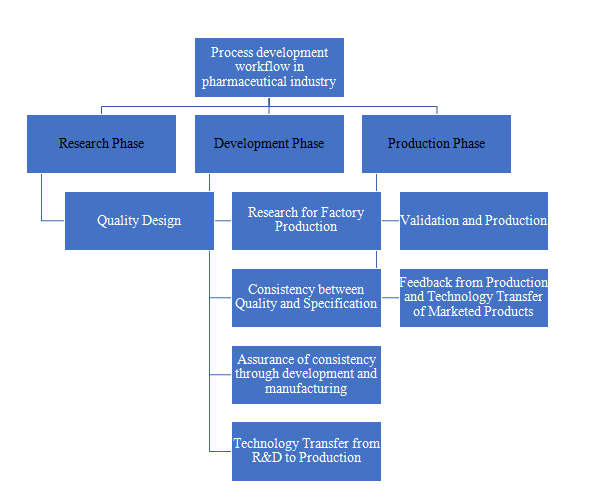
Technology Transfer’s Significance to the Pharmaceutical Industry:
- To define data needed to transfer technology from research and development to actual manufacturing by organising the numerous data gathered during research.
- To clarify the information required to transfer current product technology between multiple production facilities.
- To demonstrateparticular steps and issues with the types of technology transfer in order to promote efficient technology transfer.
- Process For Technology Transfer:
There are two ways to share knowledge and information about technology: formally, through technology transfer agreements, and informally, through the transmission of expertise.
Transferring intellectual property from one organisation to another is done through a Technology Transfer Agreement (“TTA”). TTA is used to refer to a wide range of contracts that are meant to cover the procedure of transferring ownership or the right to use a certain technology from one party to another.
The approval process for foreign technological collaboration is as per FEMA requirements, through automatic route and on approval from RBI.
The Project Approval Board evaluates the merits of any other applications for foreign technology agreements that do not fulfil the requirements for automatic approval (PAB).
Applications for such ideas should be sent to the Department of Industrial Policy Promotion, Ministry of Industry, Udyog Bhawan in Form FC/IL (SIA), according to the secretariat for industrial assistance. No fees are to be paid. It takes 4 weeks to get approval after submitting an application.
The following rules would apply to foreign financial/technical collaborators who have previously engaged in business or have ties to India:
- Those who have or had any prior joint ventures, technology transfer agreements, or trademark agreements in the same or related fields in India would not be eligible for FDI and/or technological collaboration through an automatic method.
- Investors in technology for the aforementioned category of suppliers will need to seek FIPB/PAB approval for joint ventures or technology transfer agreements (including trademarks), outlining the specific reasons why they feel it is necessary to establish a new joint venture or entera new technology transfer.
- The burden of evidence to the satisfaction of the FIPB/PAB that the new proposal will not in any way harm the interests of the current joint venture, technology/trademark partner, or other stakeholders falls squarely on such investors/technology suppliers. The FIPB/PAB shall have the exclusive power to accept the application with or without conditions or to reject it outright while properly documenting its justifications.
- Challenges Faced In Technology Transfer:
- R&D is risky & costly
- Uncertainty in the outcome and long gestation period
- Strict adherence to the time schedule
- Lack of experience in introducing first-of-its-kind productsglobally
- Unethical competition and infringement
- High patent costs.
- Opportunities In Technology Transfer:
- Creates a forum for the exchange of ideas
- Safeguards intellectual property.
- Encourages economic growth by utilising breakthrough technology for commercial purposes.
- Obtains goals through sharing and/or combining resources that neither side could achieve alone.
Conclusion
India has invested a large amount of public money in creating top-notch research institutes and providing them with enough funding. It is crucial that the technology created by these institutions be commercialised and that society or the government receives the advantages. Thus, a strong technology transfer policy is essential.
To overcome the difficulties of a fast-paced technology transfer, a thorough process map with a clear sequence of activities may be used to launch technology transfer and production. For which, businesses employ a control-tower strategy.The best practise for such transfer, the development of the knowledge-management infrastructure, and the use of digital and advanced analytics tools for transfers by assembling a team of top specialists and holding a hackathon based on current process maps. By doing this, we can respond promptly to any technological difficulty, enabling speedier transfers, faster delivery of essential medications, and ultimately aiding in the preservation of lives and livelihoods.
FAQs
What are the key regulations governing technology transfer in India?
Indian Contract Act, 1972, Competition Act, 2002, Foreign Exchange Management Act, Copyright Act, 1957, Trademark Act, 1999 and Patent Act, 1970.
What are the challenges faced in technology transfer in India?
1. Ru0026amp;D is risky u0026amp; costlyu003cbru003e2. Uncertainty in outcome and long gestation periodu003cbru003e3. Strict adherence to time scheduleu003cbru003e4. Lack of experience in introducing first of its kind products globally.u003cbru003e5. Unethical competitionu003cbru003e6. High patent costsu003cbru003e8. Infringement
What is a technology transfer agreement?
Transferring intellectual property from one organisation to another is done through a Technology Transfer Agreement (TTA). It is used to refer to a wide range of contracts that are meant to cover the procedure of transferring ownership or the right to use a certain technology from one party to another. TTA’s are essential tools for making sure that new innovations are produced via collaboration between various academic and commercial organisations in the life science industry and that current ones are utilised effectively.
[1]Item 8 of Schedule II to the Foreign Exchange Management (Current Account Transactions) Rules, 2000.
[2] https://www.meity.gov.in/writereaddata/files/National_IPR_Policy.pdf
King Stubb & Kasiva,
Advocates & Attorneys
Click Here to Get in Touch
New Delhi | Mumbai | Bangalore | Chennai | Hyderabad | Mangalore | Pune | Kochi | Kolkata
Tel: +91 11 41032969 | Email: info@ksandk.com
By entering the email address you agree to our Privacy Policy.